I always encounter assertions similar to the following quote:
The electrons in the conduction band have higher energy than those in the valence band of a semiconductor.
We know by definition the conduction band is much more further away from the nucleus comparing to the valance band.
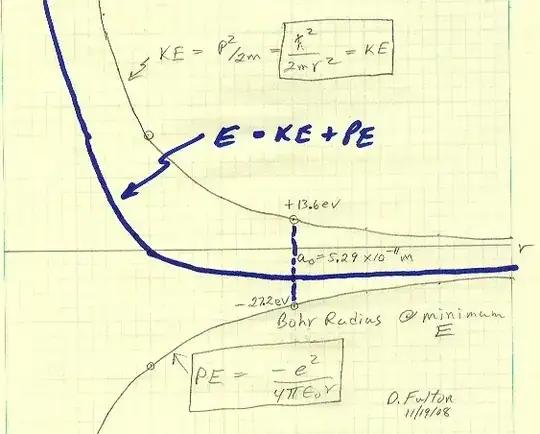
But in contrast to the quote above, below plot shows an energy of an electron versus its distance form the nucleus:
[Blue is curve shows the total energy]
The curve shows the absolute value of the total electron energy is getting closer to zero hence decreasing with distance from nucleolus.
However in band gap theory the energy is shown as if it is increasing by distance as shown here:
 [As you see above the energy increases with y-axis where y-axis also depicts the distance from mucleus]
[As you see above the energy increases with y-axis where y-axis also depicts the distance from mucleus]
Why the electron energy with respect to distance from nucleus in Bohr atom model doesn't match the one in band theory? Or am I getting something wrong here?
As you see in the equation the total electron energy absolute value goes to zero by n where n increases with distance from the nucleus.