I wish to measure microvolt-level fluctuations in a small volume of electrolyte solution, currently saltwater. My existing setup works but has some strange data so I'm hoping someone with more experience can help me sort it out.
Equipment
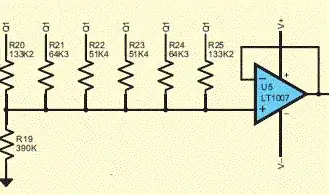
I'm using an electrically-isolated voltmeter sensitive in the ~30 uV range at a 100 Hz sampling rate. The sensor wire (red) is immersed in the solution along with the BNC ground wire (also coming from the sensor) which is then split to connect with Earth ground.
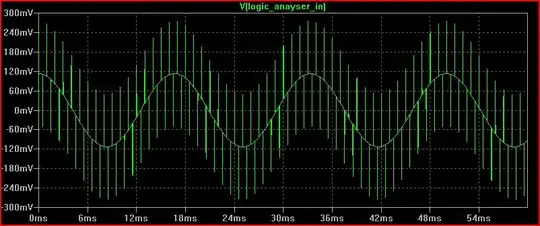
I've validated this setup by placing an additional wire into the solution carrying a 1 Hz sine wave produced by my sound card, which is picked up by the sensor as expected:
Questions
Is this circuit really doing what I expect it to? It seems to work but I don't know exactly why. Do I even need the Earth ground if everything is shielded?
When I use distilled water I get roughly the same reading (!?) and can also induce the sine wave. I didn't think distilled water was at all conductive, so... how is this possible? I've tried to carefully clean all equipment, new tube, new leads, no touching the wires, etc., although I'm trusting Arrowhead that my water is actually distilled...
When using saltwater, the voltage seems to increase slowly over time, even when EMF shielded (see chart.) I had expected it to be more constant on average or go down--- what might account for this behavior?
Thanks!