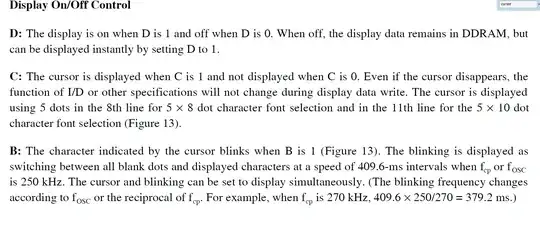
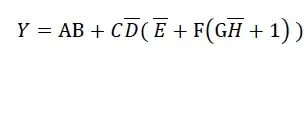There is no way you'll get a sustainable 11.5A from any normal 1.5V AA alkaline cell that I know of, due to their internal resistance, which typically seems to be about 150mΩ at room temperature.
The highest current you can expect from such a cell is under a short circuit condition, where 0V appears at its terminals, and its entire EMF of the cell is presented across its internal resistance. This would be a current of:
$$ I = \frac{V}{R} = \frac{1.5V}{0.15Ω} = 10A $$
You can't get any power from the cell in those circumstances because there's no potential difference across its terminals, so short-circuit current isn't a very useful specification. Rather you would be more interested in the greatest power you can expect from a cell, which would be when the connected load also has 150mΩ resistance. In that case, the cell has half its EMF (0.75V) across its own internal resistance, and the remaining 0.75V at its terminals, across the load. See Maximum Power Transfer Theorem. It that condition, the current through the load, and the corresponding power delivered to it is:
$$
\begin{aligned}
I &= \frac{0.75V}{0.15Ω} = 5A \\ \\
P &= IV = 5A \times 0.75V = 3.8W
\end{aligned}
$$
With 8 of those cells in series, the maximum power they can make available to you would be \$8 \times 3.8 = 30W\$, at a voltage of \$8 \times 0.75V = 6V\$.
Compare that with the power demanded by your stalled motor, to begin spinning:
$$ P = 12V \times 11.5A = 140W $$
Another way to look at this is from the motor's perspective. In a stall condition, the motor represents a near short circuit across the battery of cells. You can estimate the impedance of the motor windings:
$$ R_{STALL} = \frac{12V}{11.5A} = 1\Omega $$
Your battery of 8 cells has a total internal resistance of:
$$ R_{BATT} = 8 \times 0.15\Omega = 1.2\Omega $$
The circuit with a stalled motor across that battery looks like this:
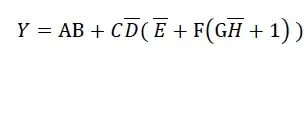
simulate this circuit – Schematic created using CircuitLab
Those 5.5A may or may not be enough to get the motor started, overcoming friction in the motor and its mechanical load, but it's a big ask from the cells.
How much would the a cell heat up with 5.5A through its internal resistance? The power dissipated in each cell would be:
$$ P = I^2R = (5.5A)^2 \times 0.15\Omega = 4.5W $$
That's quite a lot of power, and the resulting heating could quite easily damage the cell if sustained for more than a few seconds. I can only state this qualitatively, because I have no idea of the heat capacity of the cell's materials, and only you can estimate how long such conditions will prevail.
Another consideration is how long can the cells actually provide 5.5A? Internal resistance rises as the cells discharge, and to get an idea of what 1Ω across 8 cells would do over time, you have to turn to the cell's datasheet. Duracell's datasheet contains a number of graphs from which you can get an idea of what to expect from a cell. This is one of them:
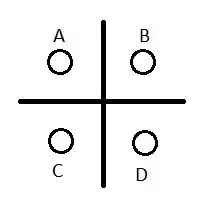
As you can see here, a cell might still produce 1V, under a load of 1A after about 1 hour. From that I will infer (very approximately) that the internal resistance has risen to:
$$ R = \frac{V}{I} = \frac{1.5V - 1.0V}{1A} = 0.5\Omega $$
in a space of one hour, or in other words at a rate of about 0.5Ω per hour. At 6 times that current, I'll guess that the cell's internal resistance will rise 6 times faster, at 3Ω per hour. This is a terribly imprecise estimation, but it serves to give us some idea, at least. I'll also guess that once the cell reaches 0.2Ω (an increase of 0.05Ω) internal resistance it will be useless to you, because it will no longer be able to provide enough stall current to get the motor started.
That gives you a useful service life for that cell of:
$$ \frac{0.05}{3} \times 60min = 1min $$
Of course this assumes a constant current draw of over 5A, which is not going to be the case. Again, without more information, I'm flying blind, but you get the idea.
Of course the normal running current of the motor will be either 0.4A with no load, or \$\frac{14W}{12V} = 1.2A\$ at full load. I'll let you work out for youself what that means for your cells.
Replacing AA cells with D cells will make quite a difference, since they have lower internal resistance, and obviously more energy. Another approach you might take is to connect two batteries of 8 cells in parallel, which will drop the combined internal resistance to 0.4Ω half of what it was. Even better, just use a bunch of lithium ion/polymer cells in series to obtain near 12V, or a single 12V sealed lead-acid battery, both of which have significantly lower internal resistance, solving your problem outright.