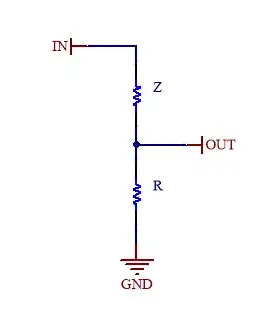
I’m trying to characterize two electrodes for ECG which are made as a classic PCB with a rounded shape and with the copper covered by a thin layer of gold (ENIG process). My first attempt was to put these electrodes in a 1% NaCl saline solution and connect them to a known load to create a voltage divider (in the picture Z represent the electrodes/solution). Then I applied a DC voltage to calculate the electrodes/solution impedance. In the schematic, Vin is the DC voltage aplied and R is the known resistance from the electrodes/solution to ground with a value of about 22.6 Kohm. The resulting value of Vout is then used to calculate the Z impedance.
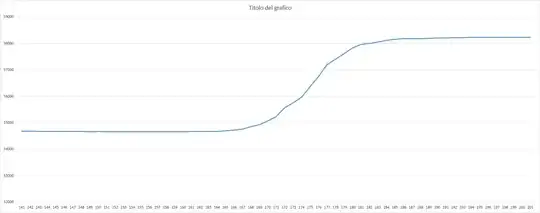
The result is the following graph which shows 5 minutes of constant voltage application (every sample represent one second).
Why I obtain this trend? I really don’t understand why there are these kind of steps during time. And on top of that, for every test I perform I have different results which means that not always the trend reach an almost stable value (like in the case shown in the picture) but sometimes, even after two hours, it continues to grow.
In the test illustrated, the "distance" between steps are successively (ohm)
2056 - 2151 - 2293 - 2347 - 2526 - 2637 - 2821 - 2969 - 3163 - 3352 - 3558
In the following picture a particular of the last step where the impedance rises about 3 Kohm in ten seconds and then stops.
Can anyone explain me the reason of this behaviour?