I understand that this is arguably a peripheral electronics problem, but it seemed to fit here better than in physics.
I've been doing some power electronics design work with large IGBTs mounted on forced-air aluminum heat sinks. To run thermal tests, we use USB thermocouple boxes and a spreadsheet to aggregate data. It takes 30-60 minutes for most of our heat sinks to reach thermal equilibrium. Given how often we run this kind of test, I decided it would be nice to add a feature to the spreadsheet which would predict the thermal equilibrium temperature early in the procedure. If we could know where a system will level out, without having to actually wait for it to do so, it could save us days of testing.
I presume that if we could take two points along the temperature curve, we should be able to extrapolate its asymptote. Note that all temperatures are assumed to be temperatures above ambient, as we are also measuring the temperature of the air intake into the fan. My derivation is as follows:
$$ t \text{: time}\\ T(t) \text{: temperature of sink above ambient at time t}\\ Q(t) \text{: energy stored in sink}\\ P_{in}\text{: power dissipated into sink (assumed constant)}\\ T(\infty) \text{: equilibrium temperature of sink for the given power input}\\ P_{out}(t)\text{: power dissipated from sink to ambient}\\ C_{\Theta} \text{: thermal capacity of sink}\\ \tau_{\Theta} \text{: thermal time constant of sink}\\ \text{From definition: }C_{\Theta} = \frac{\Delta Q}{\Delta T} \\ \text{From definition: }R_{\Theta} = \frac{T}{P_{out}} \\ \text{At equilibrium, input power is equal to output power, so this gives:}\\ T(\infty) = R_{\Theta}P_{in} \\ \text{Energy stored is power-in minus power-out integrated over time:}\\ \Delta Q = \int_{t_1}^{t_2}(P_{in}-P_{out})dt \\ \text{Input power is constant over time, so this gives:}\\ \Delta Q = P_{in}\Delta t - \int_{t_1}^{t_2}P_{out}dt \\ \text{Substitute for output power:}\\ \Delta Q = P_{in}\Delta t - \frac{1}{R_{\Theta}}\int_{t_1}^{t_2}Tdt \\ \text{Substitute for sink energy:}\\ C_{\Theta}\Delta T = P_{in}\Delta t - \frac{1}{R_{\Theta}}\int_{t_1}^{t_2}Tdt \\ \text{Multiply by thermal resistance:}\\ R_{\Theta}C_{\Theta}\Delta T = R_{\Theta}P_{in}\Delta t - \int_{t_1}^{t_2}Tdt \\ \text{Substitute for equilibrium temperature:}\\ R_{\Theta}C_{\Theta}\Delta T = T(\infty)\Delta t - \int_{t_1}^{t_2}Tdt \\ \text{Solve for equilibrium temperature:}\\ T(\infty)=\frac{C_{\Theta}R_{\Theta}\Delta T - \int_{t_1}^{t_2}Tdt}{\Delta t} $$
So if I know the thermal resistance and capacity of the sink, I should be able to compute equilibrium temperature from two arbitrarily-chosen points on the observed temperature curve, and the integral of the data between those points. However, I've run into a problem. My equations seem to be correct, but the numbers I'm getting are totally screwy.
This particular sink weighs 10 lbs. Several websites list the specific heat capacity of aluminum as being ~.9 J/(K*g), leading to a thermal capacity of approximately 4100 J/K. We ran a test to determine the thermal resistance of the sink to ambient, by putting a fixed DC current through the IGBT packs integrated diodes and allowing the sink to come to thermal equilibrium. That gave a thermal resistance of approximately .025 K/W, for our particular combination of air flow and device layout on the sink. This gives a time constant of about 100 seconds.
Again, observations show that this sink takes nearly an hour to reach equilibrium (or at least to get so close I can't measure the difference). I'm an order of magnitude off! I have a few guesses:
- This heat sink is not aluminum. Unlikely.
- The thermal capacity of the sink changes with temperature. Unlikely.
- The thermal resistance of the sink changes with temperature or some other unidentified factor, possibly the fan slowing down for some reason. More likely than the previous two, but changing by an order of magnitude still seems unlikely.
- Input power is not constant. Vce changes with temperature, but again, not by an order of magnitude.
- I've misplaced a decimal point. Always possible, but I can't find it.
- I'm totally misunderstanding the entire problem.
Does anyone have an experience in this area? Any suggestions as to what I'm doing wrong?
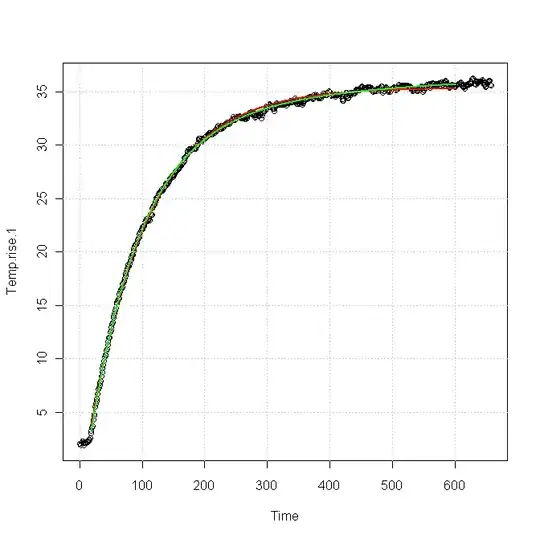
Edit: I've uploaded two example temperature curves here, for a different sink than the one I was discussing earlier. Same problems seem to occur, though.