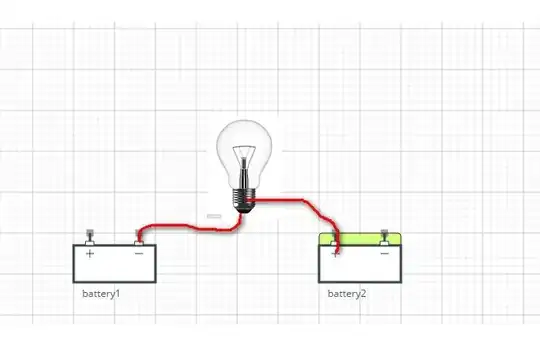
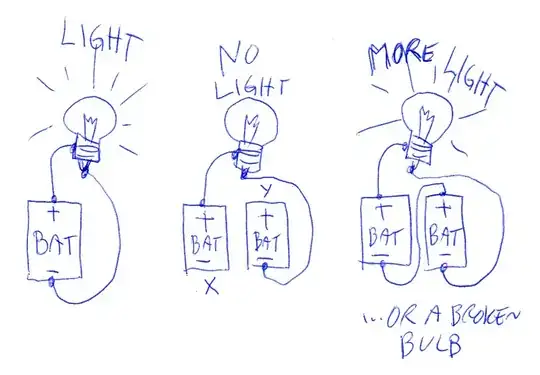
I'm new to learning about electricity. I have learned that Electrons moves from - to + when there is a voltage differential.
My conclusion is, that I can connect the plus side of one battery to a bulb, and connect it to the minus side of the second battery without closing a circuit.
This way, the electrons will move, and the bulb will light?
Is it will light for at least a few nanoseconds?
Bonus: There is an online website that can help me create the diagram and test it? or just share here the image?
Is it the truth that the bulb will be lighted for a few nanoseconds? (see answers below)