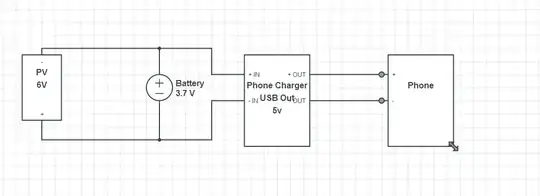
Let's say I have a budget of $15-20 to build a pH meter. I will then read the analog values from a microcontroller or such, but I want to first focus on somehow getting a reliable pH value on a shoestring budget.
First of all, is this even possible for an electronics hobbyist? I see existing pH meters out there but they are quite expensive.
There are commercial options running into the hundreds of dollars to cheap ones like these: http://www.sparkyswidgets.com/product/leophi which still require an external probe.
Ideally I want to build a pH meter circuit that has electrodes that simply dip into water to measure its pH.
I also found this: http://overskill.alexshu.com/cheap-ph-meter-hack-for-arduino/ but it's based on hacking an existing pH meter. I'd first like to hear opinions on whether a complete DIY sensor circuit is possible to do.