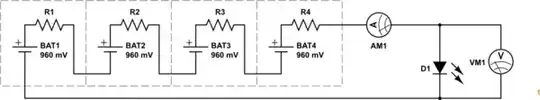
I'm working on an in-class demo / hands-on for my sons primary school class, and I've made some small batteries with New Zealand 10 cent (copper coated) coins, and zinc washers, and vinegar soaked cardboard. Each individual cell is measuring about 0.96 volts, but when I put 4 of them together, I only get out about 2.6 volts. I'm wondering if there is something I'm unaware of about the nature of these batteries that makes them not add up.
Also, even at 2.6 volts, the same voltage as I'm getting out of a pair of eneloop AA's, the LED is not very bright at all -- compared to hooking it up to the eneloop AA's, where the LED is quite bright. Is this because of the low amperage of the vinegar battery? Would putting more in series make it better (or do I need to make a second one and hook them up in parallel?).
I'm a bit of a noob with electronics, mostly learning it now as my son is very interested, so having some fun learning it with him.
Thanks for any tips.
I've attached a picture below showing the intended final product (I squeeze the top and bottom of the led wires to complete the circuit, as a simple switch). On the right is what I'm using for my cells (minus the vinegar, and without the cardboard being cut to fit the coin.)