I initially posted this on chemistry.stackexchange but didn't get any answers, so I'm reposting it here.
Long story short - we have an electronic product that is submerged in fuels (kerosene being one of them) and uses an RGB LED (click here for datasheet). Due to a sealing problem in the enclosure, kerosene has managed to get in and cover the PCBs. What's interesting is the effect that has had on the PCB. The PCB's functionality has been completely unaffected, apart from the fact that the red LED in the RGB LED module has completely stopped illuminating. We've replicated this ourselves manually by submerging 2 new PCBs in kerosene for a day and then taking them out and powering them up and seeing that the red LED stops illuminating entirely. The green and blue LEDs continue to illuminate just fine.
Examination of the failed boards shows that there are no other electrical faults. It is just the red LED that completely stops illuminating. We measured the forward voltage across each of the LEDs in the failure condition, but didn't notice any significant difference that would explain the fault.
After leaving the PCBs to dry, the red LED starts working again. So the problem is not permanent.
Looking at the last page on the datasheet, the LED material is listed as AlGaInP / GaAs. Is there any obvious reaction between kerosene and these materials that would explain why just the red LED stops working?
Update 1: I've carried out the following experiments:
- Dripping kerosene on to the LED.
- Submerging the PCB+LED in kerosene while running.
(Videos to follow up later on today, hopefully)
In both cases, there was no perceived effect on the LED - it continued to operate just fine. This would seem to indicate that the problem is not purely an optical problem between the kerosene and the LED. So far, the problem has only occurred after soaking the LED in kerosene for some time.
Update 2: I've taken a fresh PCB with LED on it (haven't done any tests yet with just the LED) and soaked it in kerosene. I've taken some close up photographs of the LED before soaking, after soaking while it's not working and after it resumes working after it's been left to dry.
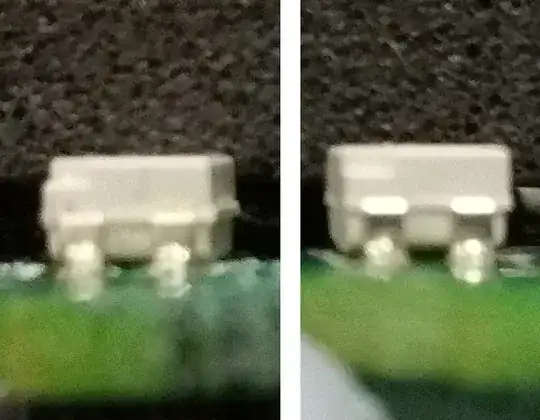
What the photos show is that there is a very obvious bulge in the LED lens during the period when it's not working. Once the bulge recedes, the LED illuminates again.
Unfortunately, I don't have a camera set up on the PCB to see the exact moment that it stops working. I'd let it soak for about an hour before it stopped working. I checked on the LED every now and then and noticed no change in the LED brightness. I came to check it once and it was just off. My suspicion is that the change is sudden.
Judging by the swelling, I'm going to guess that there is some mechanical damage internally that's moving something and once the swelling recedes it springs back to position.
Left: Kerosene-soaked LED; Right: Normal LED
LED in failed state after soaking
Left: Kerosene-soaked LED after being left to dry and in the working condition; Right: Normal LED
Kerosene-soaked LED after being left to dry and in the working condition