So, I'm working on a way to produce PCBs for small jobs, and I thought that lasers might be a good way to go, as etching seems to be very difficult from small traces, needed for many microcontrollers.
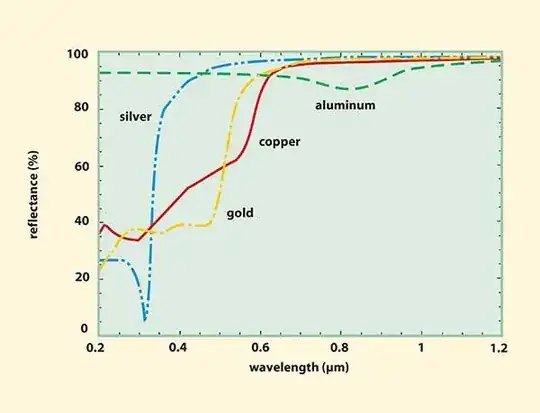
I started by searching the absobance spectrum of copper, as the metal itself is very reflective. A quick search turned out that the absorbance of copper rests right around 800nm. Thus I came to the conclusion that an 808nm etching diode would probably be best.
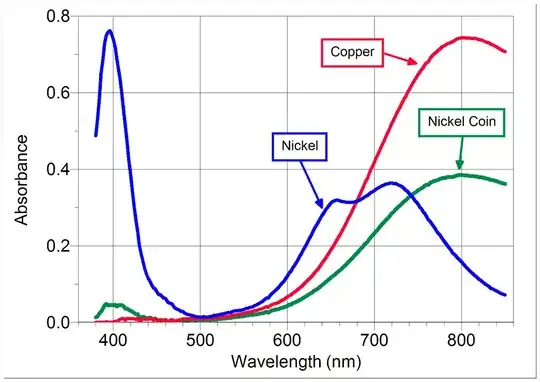
My question to you, is weather or not the laser qould actually remove material, or if the copper will take on the heat? 808nm lasers are very focusable,and I plan on having an estimated power of 360KW/cm2 (40W diode at .112mm2 dot)..
I've worked with many lasers before, ranging from IR to UV, and I know enough safety to know that 808 modules are generally beasts.