42/58 Tin / Bismuth is not unknown as a low temperature solder but has issues.
While widely used for some very serious applications (see below) it is not a mainstream industry contender for general use. It is not obvious why not given its substantial use by eg IBM.
Identical to the Bi58Sn42 solder you cite is:
Indalloy 281, Indalloy 138, Cerrothru.
Reasonable shear strength and fatigue properties.
Combination with lead-tin solder may dramatically lower melting point and lead to joint failure.
Low-temperature eutectic solder with high strength.
Particularly strong, very brittle.
Used extensively in through-hole technology assemblies in IBM mainframe computers where low soldering temperature was required.
Can be used as a coating of copper particles to facilitate their bonding under pressure/heat and creating a conductive metallurgical joint.
Sensitive to shear rate.
Good for electronics. Used in thermoelectric applications.
Good thermal fatigue performance.
Established history of use.
Expands slightly on casting, then undergoes very low further shrinkage or expansion, unlike many other low-temperature alloys which continue changing dimensions for some hours after solidification.
Above attributes from the fabulous Wikipedia - link below.
According to other references it has low thermal conductivity, low electrical conductivity, thermal embrittlement issues and potential for mechanical embrittlement.
SO - it MAY work for you, but I'd be very very very cautious about relying on it without very substantial testing in a wide range of applications.
It is well enough known, has obvious low temperature advantages, has been widely used in some niche applications (eg IBM mainframes) and yet has not been welcomed with open arms by industry in general, suggesting that it's disadvantages outweigh advantages except perhaps in areas where the low temperature aspect is overwhelmingly valuable.
Note that the chart below suggests that flux cored versions seem to be specifically unavailable either as wire or as preforms.
Comparison chart:
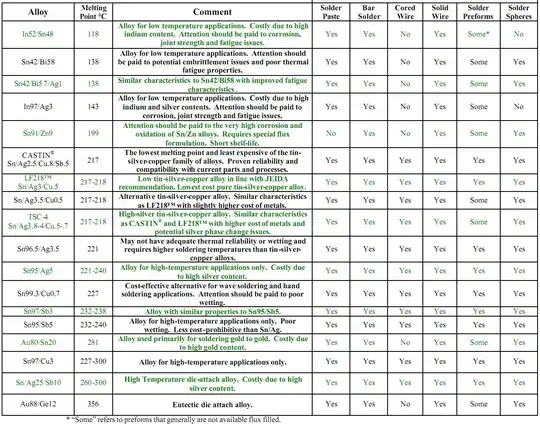
The above chart is from this superb report which however does not provide detailed comment on the above issues.
Wikipedia notes
- Bismuth significantly lowers the melting point and improves wettability. In presence of sufficient lead and tin, bismuth forms crystals of Sn16Pb32Bi52 with melting point of only 95 °C, which diffuses along the grain boundaries and may cause a joint failure at relatively low temperatures. A high-power part pre-tinned with an alloy of lead can therefore desolder under load when soldered with a bismuth-containing solder. Such joints are also prone to cracking. Alloys with more than 47% Bi expand upon cooling, which may be used to offset thermal expansion mismatch stresses. Retards growth of tin whiskers. Relatively expensive, limited availability.
Motorola's patented Indalloy 282 is Bi57Sn42Ag1 . Wikipedia says
- Indalloy 282. Addition of silver improves mechanical strength. Established history of use. Good thermal fatigue performance. Patented by Motorola.
Useful lead free solder report - 1995 - nothing to add on above subject.
