Electrons are present in every atom.
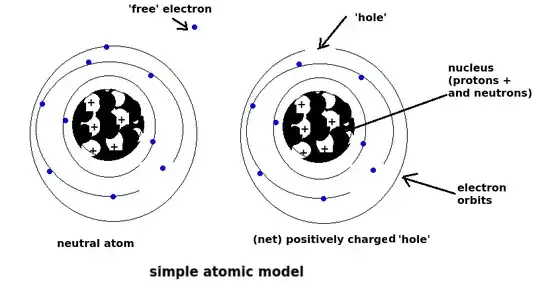
In materials such as metals (good conductors) the outermost electrons are very loosely bound to the nucleus and tend to wander around randomly in the material matrix leaving 'holes' in the normally neutral atom's electron structure.
These 'free' electrons take their energy from thermal energy. Any individual electron (negative charge) wandering by a positively charged 'hole' will be attracted and may fill the gap. It may then sit there for a while and move off. Its a very random/chaotic process.
The result is that the material stays electrically neutral even though billions of free electrons are randomly wandering about in all directions at any given time. There is no net drift in a particular direction.
In a battery we produce a potential difference or voltage by accumulating and maintaining a surplus or decifit of electrons at the terminals. Note that the charge is produced in pairs so there always equal amounts of electrons and holes.
One terminal is positive (electron deficit, hole surplus) and the other is negative (electron surplus, hole deficit). This accumulation and maintenance of excess charge requires energy provided by a chemical reaction inside the battery.

When a circuit is made current flows almost instantly. This depends upon the wire but is somewhere between 0.5 and 0.9 times the speed of light.
This cannot be the physical (drift) movement of electrons due to the applied electric field as the drift speed of electrons is much (much) lower (see https://en.wikipedia.org/wiki/Drift_velocity). It must be an electro-magnetic wave.
A thought experiment: Imagine at the negative terminal end of the battery one (excess) electron moves from the terminal into the wire . This minute pulse of current (change in electric field) is transmitted through the wire at near the speed of light and pushes one electron out of the wire into the positive terminal of the battery to retain the overally electrical neutrality (balance) of the circuit
Now imagine this happening to billions of electrons. The result is what we term current.
The force that moves the electrons is an electric field produced by the voltage difference between the terminals and the basic rule is 'like charges repel, unlike charges attract'.
Electrons will be repelled by the negative voltage and attracted by the positive. Note that the electron does not need to travel through the wire, it only needs to move a small distance.
In terms of current direction we still tend to think that current flows from positive to negative (because that's what we teach in schools), this was due to an historical mistake made by Benjamin Franklin who simply got it wrong.In reality it doesn't matter as long as you are consistant in any calculations.
This loss and accumulation of electrons has the effect of reducing voltage at the battery terminals. It unbalances the chemical reaction inside the battery. The chemical reaction then tries to rebalance this by producing extra pairs of charge (electron + positive ion) to maintain the voltage at the terminals.
The more current that is taken by the circuit, the faster the reaction has to take place in the battery and the quicker the battery will be depleted. Measuring the voltage at the terminals you will see this drop as more current is taken.
For calculation purposes we think of a battery having an 'internal resistance' It doesn't have a real resistor inside, it just has a physical limit as to how quickly it can replace the neutralised charge pairs.
Rechargeable batteries can have their chemical reaction reversed by sending a current into the battery. The energy provided is stored in chemical form.
Fuel cells can be be provided with external chemicals to provide power. e.g hydrogen fuel cell - air (oxygen + nitrogen etc.) + hydrogen = electricity + water (plus nitrogen etc.)

