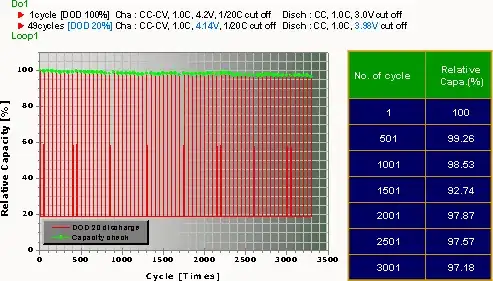
I have read recently that frequent top-off charging of my personal electronics is hard on the batteries. Specifically, Li-ion batteries in recent model smart phones and laptops will show significant loss of capacity after (very roughly) 300 cycles between the remaining charge levels of 90% and 100%.
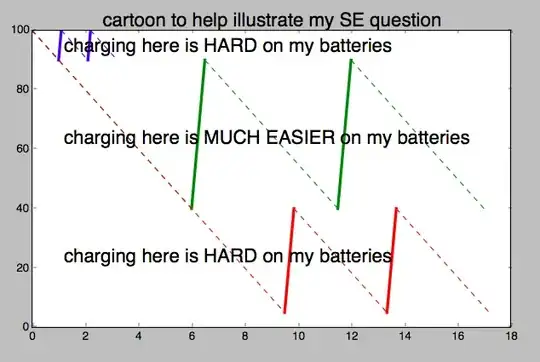
Below is a 'cartoon' of a plot to help clarify my question. Is cycling between the 90% and 100% points 'harder' on these types of batteries than an equal number of cycles between the 40% and 90% levels?
By 'harder' I mean a significant loss in battery capacity after (very roughly) 300 cycles, versus maybe 3,000 cycles.
Also, is cycling through the lower end (say 5% to 40%) also 'harder' than in the mid-range?
I'm not looking for opinions or best guess - I need a fairly clear answer with a link to further reading, and ideally an actual plot of this phenomenon - model or data.
edit: assume the charging is managed by the built-in electronics according to manufacturer's specifications.
NOTE: This question requires an engineering answer, but let's assume I am not so much of an engineer (anymore) that I have actually taken my phone and laptop apart and I'm hooking up volt meters to the individual cells!!!
Please assume I am simply either plugging into a power source, or not plugging into a power source, the way most people do, based on the 0-100% scale that my personal consumer electronic device displays.
Thanks!