I am trying to calculate Li-ion/LiPo battery's energy efficiency based on its internal resistance (as far as I see from scientific papers, the battery's internal resistance rises with its ageing, meaning that the efficiency must decrease).
Discharging: I use the most basic equivalent circuit and load:
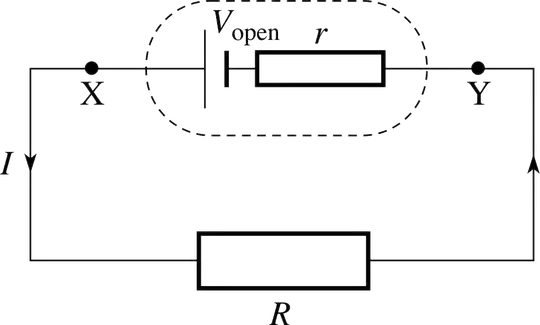
If I assume that the current is constant (the way they describe it here), then I get:
ef = (Vopen-Ir)/Vopen (probably I should integrate this by time)
(I have reduced the current and the charging time in both parts of the fraction).
By the same method, the charging efficiency will be: V/(V+Ir)
Therefore if we want to calculate the round-trip efficiency (output power/input power), it will be the multiplication of the two, giving (V-Ir)/(V+Ir)
Am I doing this right? Does it mean that for every charging/discharging current, the efficiency is going to be different?
I guess there are other factor affecting the energy efficiency, although I haven't quite found any formulas. How significant are they in comparison to internal resistance?