When you test a battery, you have to put a load on it, otherwise the voltage floats up much higher than it should, considering its remaining life.
In the high-demand application, the internal resistance of the battery becomes much more of a factor in what voltage the battery can deliver, leading the battery to reach its cut-off voltage much too early.
Let's use a camera flash as an example, for that is particularly high-demand application.
Especially if you're using your camera in sub-zero temperatures, where the internal resistance increases and the battery chemical reaction proceeds at a slower pace, you'll use up batteries incredibly fast. And those used-up batteries will be considered by the camera to be "dead", for its application, in that cold setting.
But take those "camera-dead" batteries back inside, and let them warm up, and they will indeed still have much of their life still remaining, and will even present a decent voltage as well, even under test load.
There are lots of high-demand applications. Toys or anything motorized, and also poorly designed products, which I see all the time, poorly designed in a variety of ways. But even in the standard scenario, almost everything cuts off at or above 0.8 volts, leaving energy down to 0.5 volts left to be used for the low-power-demand application and some kind of boost converter.
To sum up, the key to understanding this issue is realizing that a cell considered "dead" for the high-demand application will not be considered dead for the low-demand application, but that energy may be inaccessible without some kind of boost converter.
Also key is understanding that low-demand applications may cut-off due to voltage when there is really plenty of energy left in the batteries, which is where the voltage-booster, and I believe the Batteriser product too, if it is quality, will definitely prove useful. So, then, low-power-demand products that cut-off on a low-voltage-basis because they DO NOT have a boost, will DEFINITELY benefit from the boost.
A simple cheap LED flashlight is a good example of both a low-demand application, and a device which cut's off based on voltage, because the cheap LED flashlight uses a resistor and the forward-voltage drop of the LED to decide the cut-off.
So, for a typical 3-cell flashlight, 3x1.5=4.5 volts new. The LED drops about 3 volts. So the natural voltage cutoff for a cheap LED flashlight is actually fairly high, at 3-volts/3-cells = 1 volt per cell.
But lighting those LED's is actually a fairly low-demand application. There is definitely plenty of energy left in those cells.
So, this is the perfect example of when it WOULD be beneficial to use a boost circuit to get the remaining energy out of these cells which have only been used down to 1 volt per cell.
I watched the treatment that Dave of EEVblog gave to Batteriser, and I think that he has perhaps overemphasized where Batteriser was wrong, but may not have sufficiently thought out the above things that I relayed, as I have studied the Joule Thief extensively, and I don't think that Dave has done this. I do understand the points Dave raised, and some may still be valid concerns, but I use my Joule Thief circuits all the time, and I can attest that they are definitely beneficial, just as would be any decent boost alternative.
Finally, in an emergency, the boost products, whether Joule Thief or Batteriser, or another product, would come in handy and even may become critical in a Hurricane Florence or other disaster scenario. Sometimes just having a working flashlight is essential, and if having a Batteriser or two lying around allows me to do that, then on that additional count as well, I call Batteriser and the Joule Thief, beneficial.
===========
Edit #1
To answer an asked question, I have absolutely no affiliation with Batteriser, Batteroo Boost, or the company Batteroo, or anyone in it -- just great fondness for the Joule Thief and trying to provide them to the third world, where they can't afford electricity, or batteries, and I don't want the overinflated claims of Batteroo to torpedo the Joule Thief.
To back up what I said, I am going to appeal to Dave of EEVblog and a research study paper he referenced directly.
In his EEVblog post "The Batteriser Explained" (a fairly thorough treatment of the subject in my opinion, and worth reading), Dave states:
HERE is some great research on used batteries. About 33% is
wasted based on their data.
I appreciate Dave saying this, because it states that there really is energy left to use in the average discarded battery. He also states the following, which to me, means that the Batteroo product is still useful (just not as useful as they exaggerated):
I’m genuinely baffled as to why Batteroo would need to resort to
claims like 8 times life. This thing would still sell like hot cakes
if they claimed realistic practical figures. 50% increase in your
battery life? – great, countless people would still buy it at the
super low price point it’s at...
This study Dave references helps very much to answer this particular stack exchange question, so, to show some of their due diligence, here's the test flow chart:
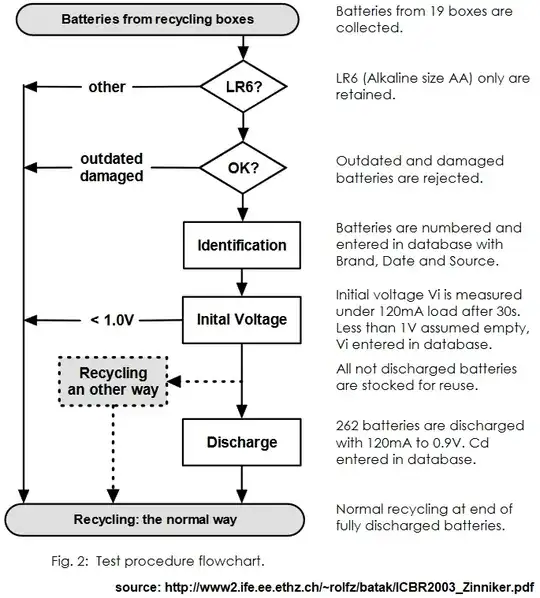
And here is a scatter plot and curve fit that shows the individual data points and that there is a nice correlation:
This chart shows how much actual capacity was left for many actual discarded batteries.
For their tests, they collected discarded batteries from 19 recycling boxes, then separated the batteries into 5 voltage classes spanning 0.1 volt from 1.1 volt to 1.5 volts. Batteries were randomly selected and discharged using a 120mA constant current load down to 0.9 volts. In the 636 battery study, 265 were discharged down to 0.9v to determine remaining life (mAh). According to their test results for discarded batteries:
- About 10% can be considered new (see 1.58v data point, fig 4 above)
- About 30% have more than 50% of their energy left
- About 40% are fully discharged (defined in study as less than 1 volt)
And lest you think that 1 volt is completely discharged because of their study, they also say:
...all batteries with an initial voltage of less than 1.0V are
registered as 0V and assumed fully discharged. Of course this is for
most of them not true, they still contain a small remaining capacity
which could be used to power low power devices (e.g. clock, or small
radio). This was not considered important in our work.
They then go into reasons why people throw away batteries with so much (>=30%) energy left:
- High Power devices (early cut-off)
- Ensure Batteries are Good (replace for each use)
- No (or bad) Battery Tester (unknown state-of-charge)
My most common personal reason is "Ensure Batteries are Good". I have an audio recorder, and don't use it that often, but when I do, I want to make sure that it doesn't fail in the middle of something important (a child's recital). So, my default action is to just put new batteries in.
The bottom line that I want to relay is, don't let Batteroo's inflated claims ruin the truth -- that there really is energy left in discarded batteries. Just watch out for leakage, because the lower the discharge, the higher the pressure.
There definitely is benefit to using a voltage booster (such as Batteroo Boost or a Joule Thief) on “dead” batteries.

