This is an FDA Class II device. It's regulatory pathway to the market is known as the 510K process, which means that Omron must demonstrate "substantial equivalence" to a device or devices that is already FDA approved that functions in largely the same manner.
Note that it is regulated under the CFR 870.2770 as an impedance plethysmography device (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=870.2770), which the Code for Federal Regulations says is " a device used to estimate peripheral blood flow by measuring electrical impedance changes in a region of the body such as the arms and legs". The device is passing a current through the body (at a certain frequency), and using this current to assess the resistance of the body, which they claim is related to body fat.
The FDA's letter of determination of the 510K application (or possibly that of a very similar Omron device) is at http://www.accessdata.fda.gov/cdrh_docs/pdf4/K043060.pdf. I couldn't find the application itself to determine what device that Omron claimed equivalence to. The claimed indications for use are:
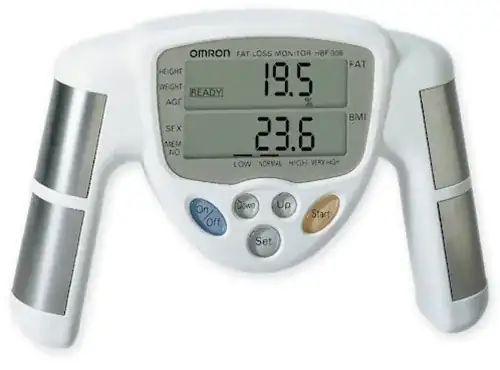
The device is a non-invasive bio-impedance analyzer
with scale for use in estimating human's body fat
percentage by calculation using the individual's BMI
(body mass index) and determination of body weight.
The OMRON HBF-400 is intended to be used by
healthy individuals between the age of 10 to 80 years.
https://en.wikipedia.org/wiki/Body_fat_percentage#Bioelectrical_impedance_analysis shows that impedance plethysmography is a valid method for assessing body fat (and offer appropriate citations in the scientific literature, which you can track down), but other factors, such as hydration status, can also alter the readings.
In any case, the FDA regulates ALL the marketing claims and Indications for Use that the applicant makes. Unlike the EU, which largely regulates safety, and efficacy can be more caveat emptor, the FDA regulates safety and efficacy, which means that the FDA considers the claim that this device measures body fat to be valid.
If a person uses this device to track personal body fat, and it is used in the same manner and conditions every time, I'd say it can do a fair job. I'd put big error bars around the actual percentage, but I'd rely on it to detect trends.
The manual is at http://www.omron-healthcare.com/data/catalog/3/107/1/IM-HBF-306-E-04-10-2011%20EN.pdf. As a Class II device, the FDA has reviewed this manual. As I suspected, they give pretty involved directions concerning how to use the device to get a valid reading.
When asking about "accurate enough??" the context must be "accurate enough for what?" If BMI reported is 2% off, nobody dies, nobody gets sick, nobody's heart stops. If you need to know if your diet and exercise regimen is working over long periods of time, it's probably good enough if used carefully and as directed.
As for "all those parameters" -- it provides two: %fat and BMI. I believe the device is integrated with a scale, and BMI is just a function of %fat and total weight.