I don't have much knowledge on LED lights and find the technology quite fascinating (yes, I know, it is not that new!)
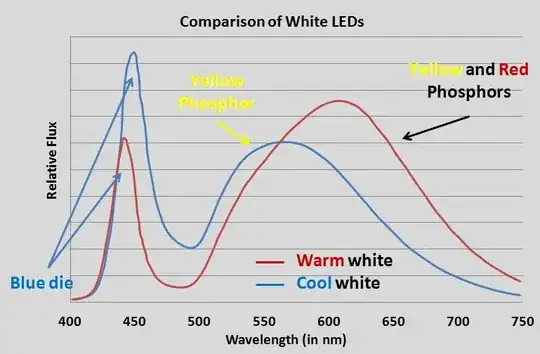
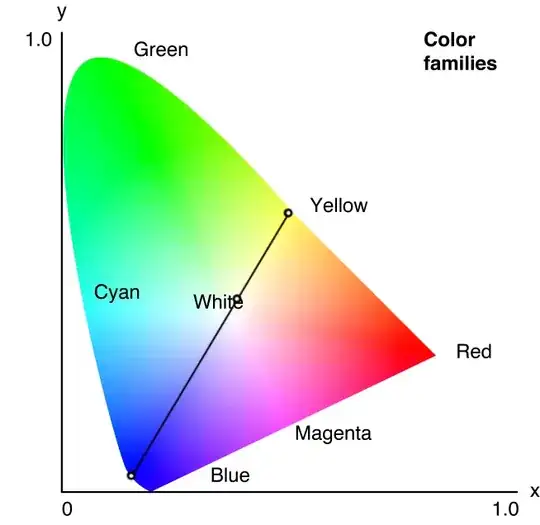
I have read that in order to create "white" light, LEDs actually need to emit light from all spectrums.
My question(s):
- How do LED light bulbs (cool white or warm) generate white light?
- What would be the light spectrum for cool white LED light bulbs?
- What would be the light spectrum for warm white LED light bulbs?
Thanks!
P.S. This is my first question here. If it should be forwarded to another SE site, please let me know!
P.P.S. I have done my research BEFORE asking but could not find a technically accurate answer.