I've tried to create this quite simple circuit.
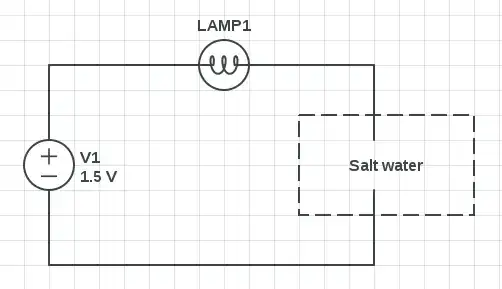
- V1: 1.5 v (AA battery)
- LAMP1: 1.5 v, 0.2 a (E10 incandescent light bulb)
- "Salt water": 10 ml regular water + 1 g salt
When I connect the wires together, the lamp lights up. When I connect the wires to various conductive materials (a spoon, tin foil, a penny,...), it works as well. But if I put the wires into a tiny bucket containing (salt) water, it does not.
Could you please explain why? (I guess that it is related to the water resistance/conductance but how exactly and how do I calculate it?)
Thank you.